Abstract
Background: The efficacy and clinical benefit of BV in patients with CD30+ relapsed/refractory (R/R) classical Hodgkin lymphoma (cHL), cutaneous T cell lymphoma (CTCL) [mycosis fungoides (MF) or primary cutaneous anaplastic large cell lymphoma (pcALCL)] and systemic anaplastic large cell lymphoma (sALCL) have been shown in Ph2-3 pivotal studies (NCT00848926, NCT01578499, NCT00866047). However, the evidence of BV use as a retreatment option is scarce. There are only two studies with available data on the effectiveness and safety of brentuximab vedotin (BV) in the retreatment setting. This research aims to investigate the effectiveness and safety of retreating these populations with BV in the real-world setting in Spain.
Methods: Non-interventional, retrospective, multicenter, medical chart review study conducted in 30 public and private Spanish sites. The study included patients aged ≥18, with confirmed diagnosis of cHL, CTCL (MF and pcALCL) and sALCL, CD30 positive, previously treated with BV as monotherapy or as a combination with another antitumor agent, with evidence of objective response and subsequent disease progression, who received ≥2 doses of BV as retreatment and a minimum of ≥6 months of follow-up, discontinuation due to toxicity, or until death.
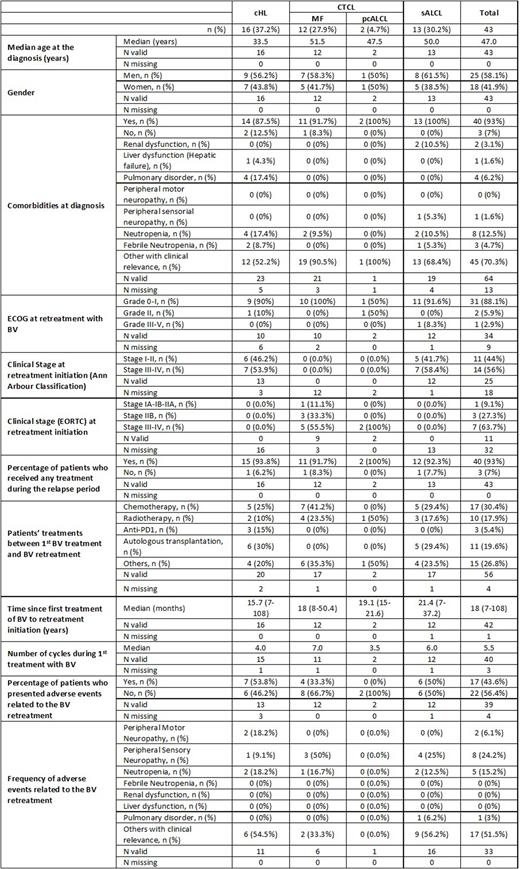
Results: Of the 51 patients enrolled, 43 were eligible for this preliminary analysis (Table 1): 16 with cHL, 12 with MF, 2 pcALCL and 13 with sALCL. The median age of the total population was 47 (33.5-51.5) years, and 58% were women. At retreatment, the Eastern Cooperative Oncology Group performance status (ECOG PS) of the patients was poorer: 18 (52.9%) had ECOG PS of 0; 13 (38.2%) had ECOG PS of 1, 2 (5.9%) had ECOG PS of 2, and 1 (2.9%) had ECOG PS of 3, and 40 patients (93%) presented with comorbidities (Table 1). The median time from the first treatment with BV to the retreatment initiation was 18 (7-108) months, with at least one treatment before BV retreatment in most of the patients (n=40; 93%) (Table 1). At initiation of the retreatment period, more than 50% of patients presented advance clinical stage (III and IV). 27 patients (67.5%) discontinued/terminated retreatment with BV after a median of 5.5 (3.5-7) cycles. The median time from end of BV retreatment until last available follow-up was 8 (6-12) months and 73% of patients were alive. 10 patients were exitus and 43.6% presented with adverse events, mainly peripheral sensory neuropathy (24.2%) (Table 1).
Conclusion: This chart review is the first Spanish study to collect information on BV retreatment in patients with CD30+ malignancies, providing information of clinical characteristics and treatment patterns in real-word practice. As BV may become established as a preferred first-line therapeutic option for both untreated stage IV in cHL and sALCL, future research efforts could be aimed at further exploring the efficacy and safety of BV retreatment in patients who progress or relapse after receiving frontline BV treatment. Further analysis of this study will be provided at the congress to determine efficacy and safety results associated to BV retreatment in the Spanish population at clinical practice.
Disclosures
Sureda:Astra Zeneca: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; MSD: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria; Jannsen: Consultancy, Honoraria; Pierre Fabre: Consultancy, Honoraria; GenMab: Consultancy, Honoraria; Kite: Consultancy, Honoraria. García-Sanz:Janssen: Honoraria, Other: Travel support, Research Funding; BeiGene: Honoraria, Other: Travel Support; Gilead: Honoraria, Research Funding; Astellas: Honoraria, Research Funding; Amgen: Honoraria; Takeda: Honoraria, Research Funding; GSK: Honoraria, Other: Travel Support; Astra Zeneca: Honoraria; In Vivo Scribe: Patents & Royalties: Indirect perception, Euroclonality primers; Novartis: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal